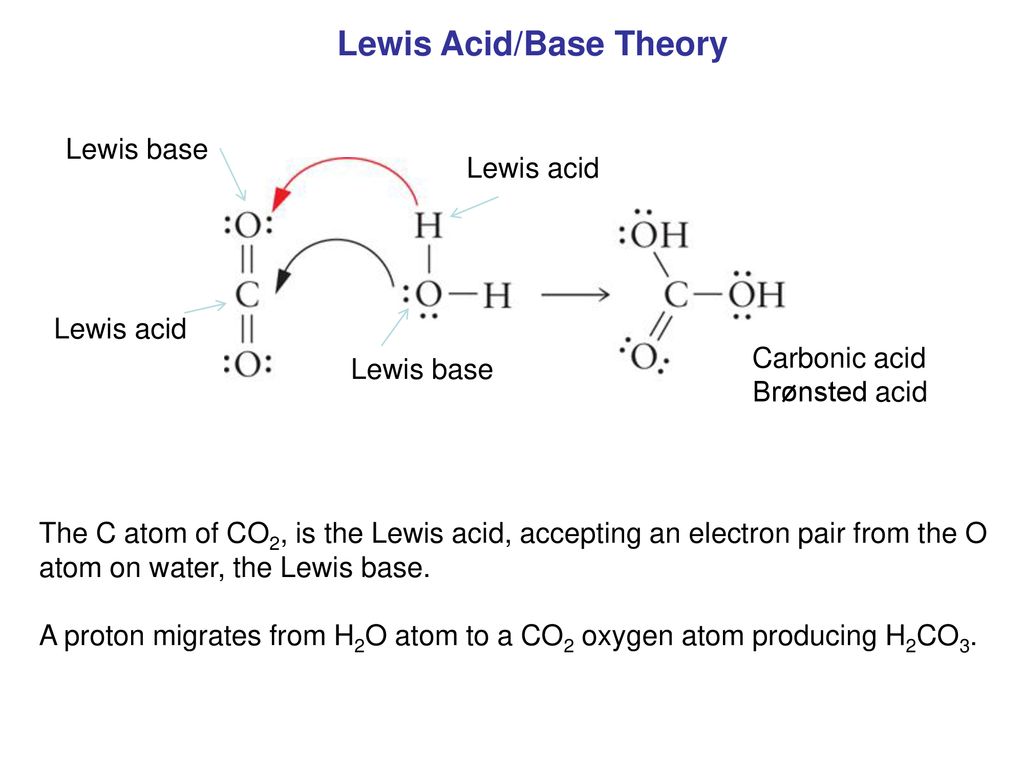
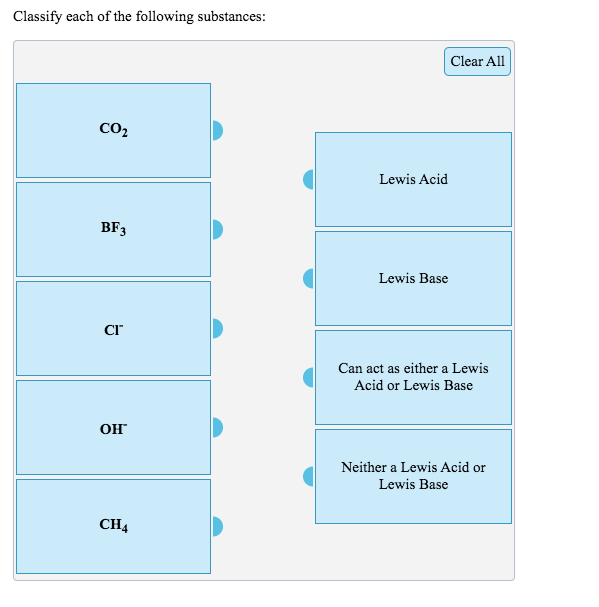
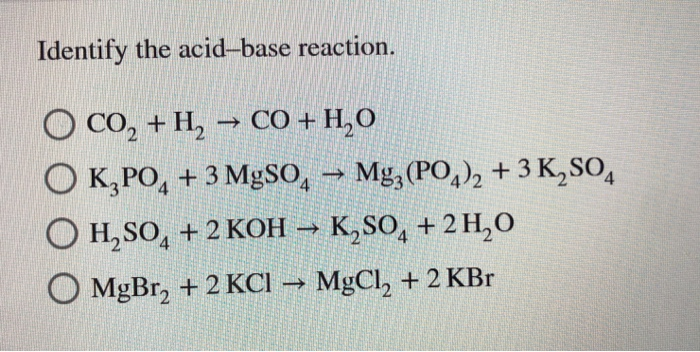Role of the Surface Lewis Acid and Base Sites in the Adsorption of CO2 on Titania Nanotubes and Platinized Titania Nanotubes: An in Situ FT-IR Study | The Journal of Physical Chemistry
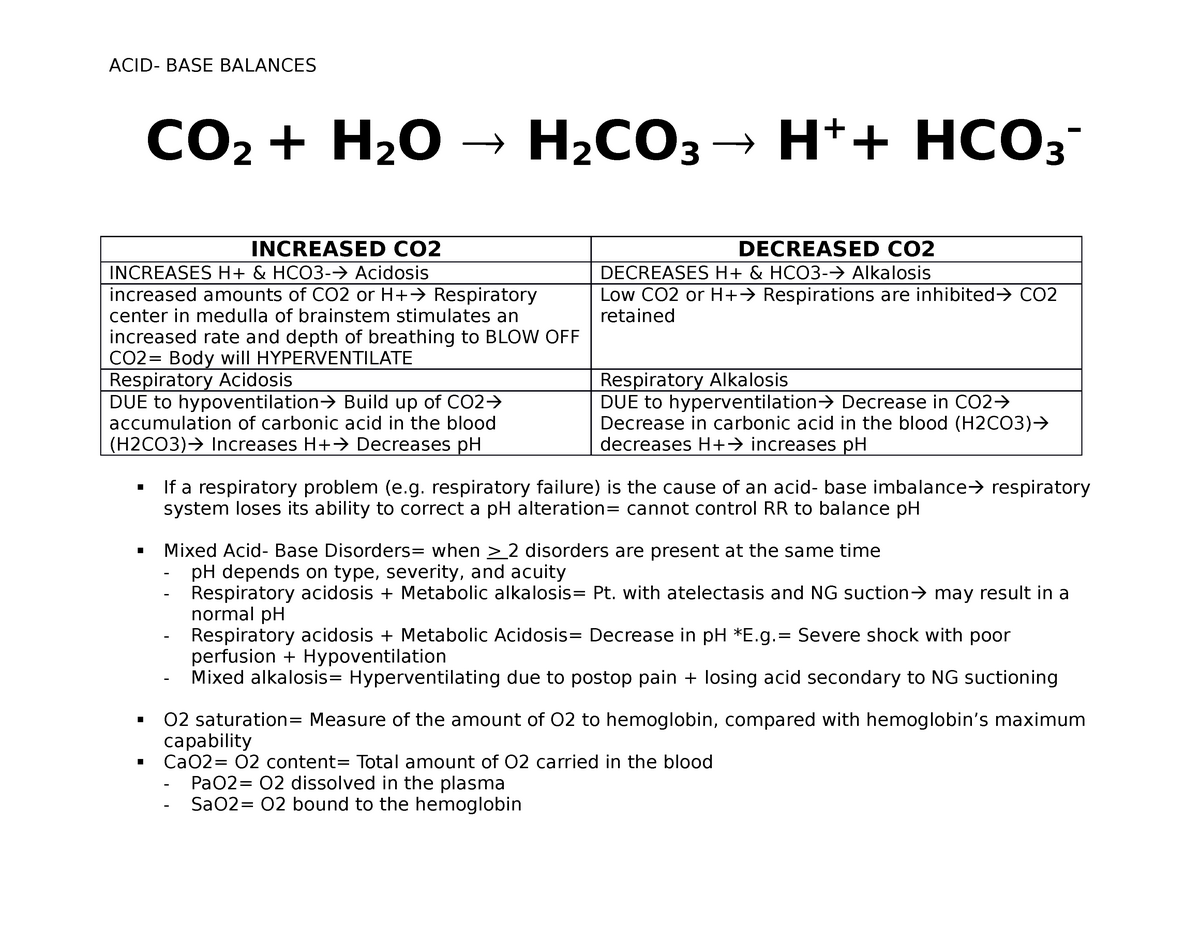
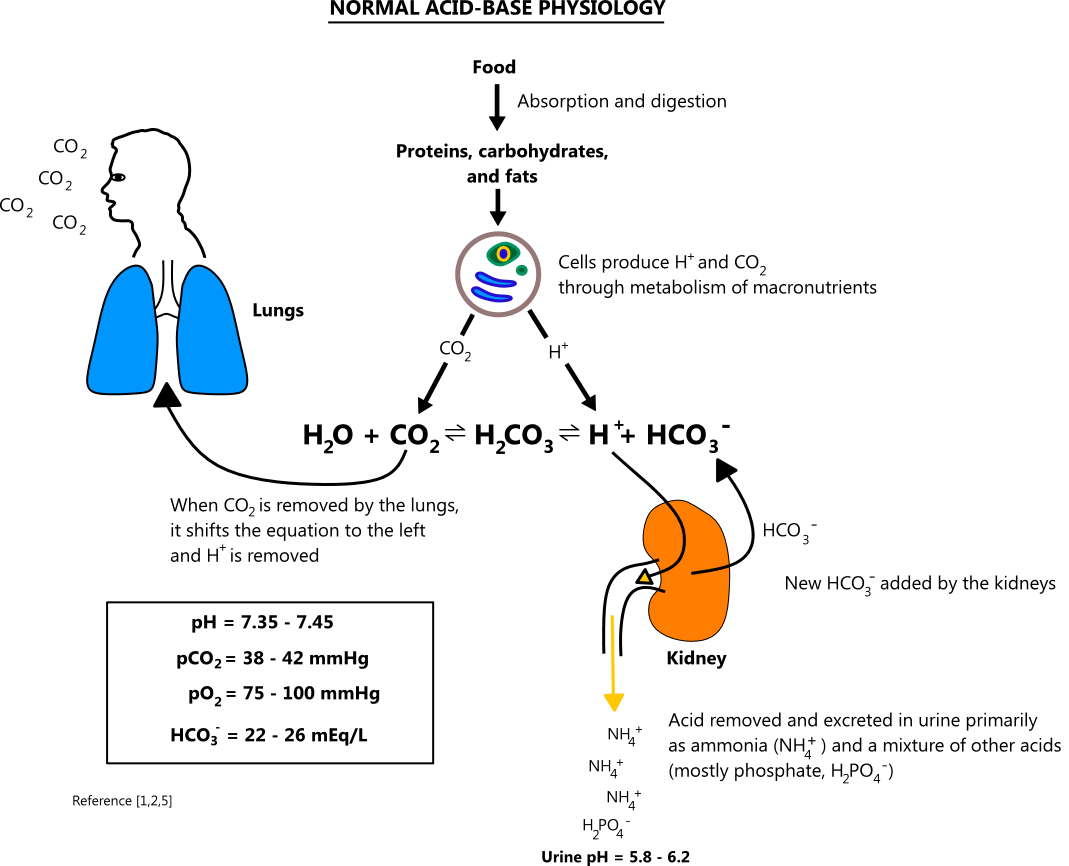
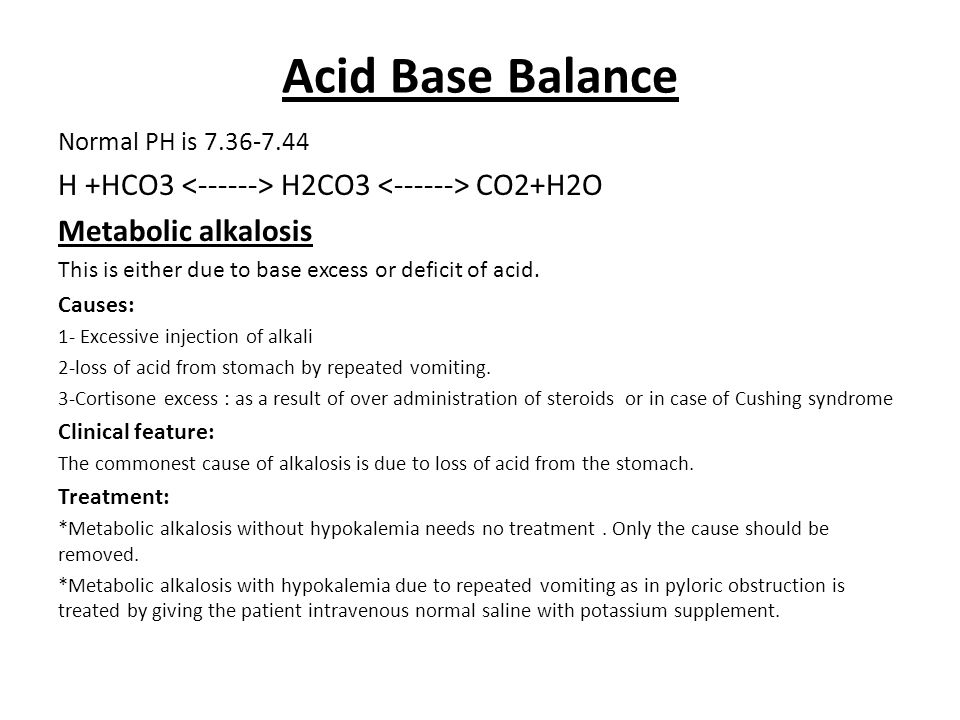
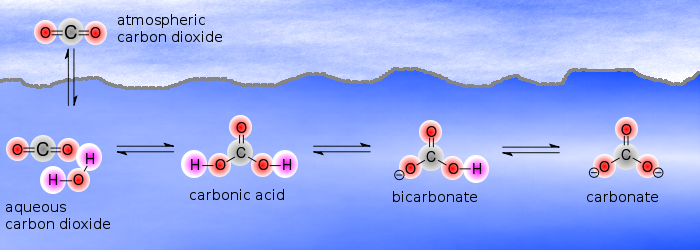
Acid- Base Imbalances - Lecture notes 1 - CO 2 + H 2 O ® H 2 CO 3 ® H + + HCO 3 – INCREASED CO2 - Studocu
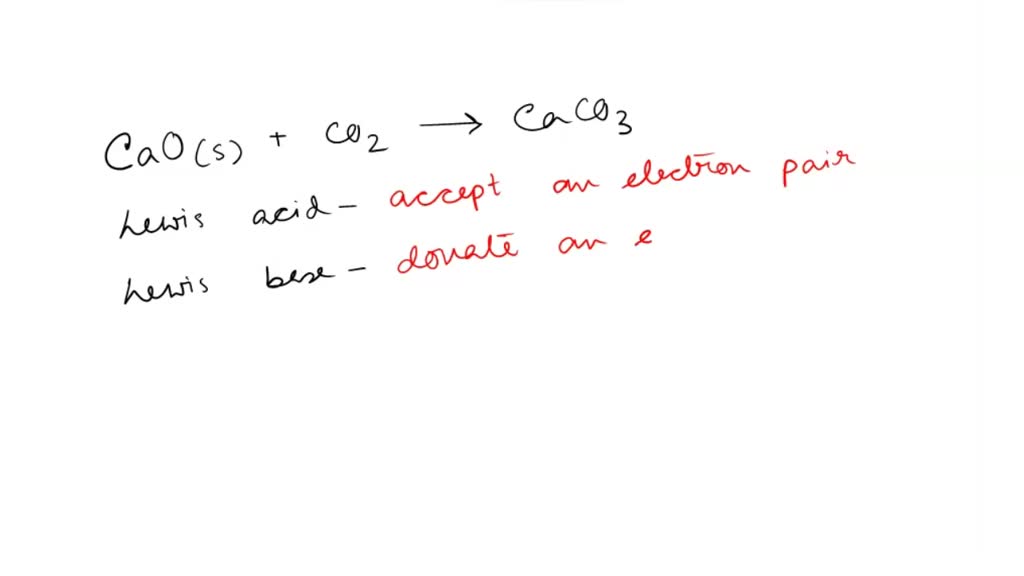
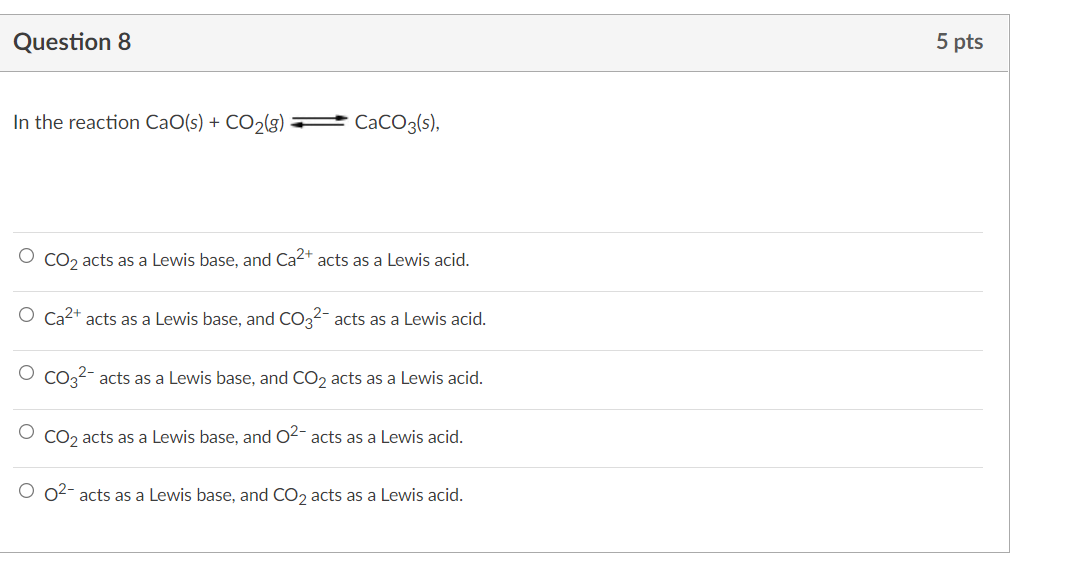
SOLVED: QUESTION 1 In the reaction: CaO(s) + CO2(g) â†' CaCO3(s), Ca2+ acts as a Lewis acid and CO32- acts as a Lewis base. CO2 acts as a Lewis acid and CO32-

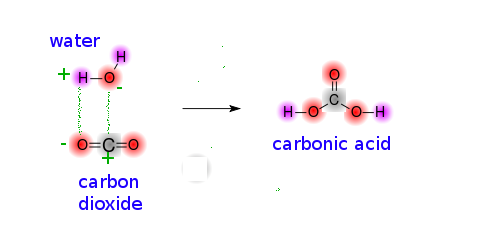
Modifying lewis base on TiO2 nanosheets for enhancing CO2 adsorption and the separation of photogenerated charge carriers - ScienceDirect